Research & Development at Nielsen Biosciences
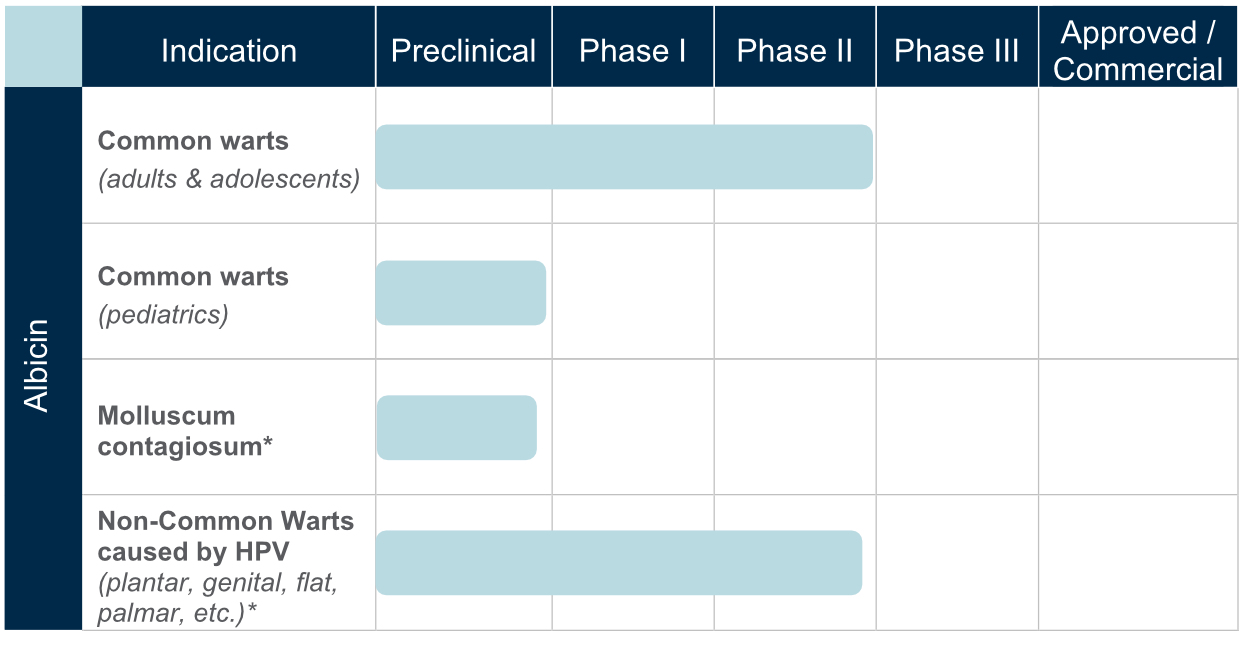
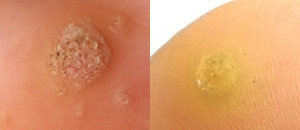
The active ingredient in our CANDIN product, currently approved as a skin test antigen for the assessment of cellular hypersensitivity to Candida albicans, is currently under investigation for the treatment of Verruca vulgaris or common warts. The antigen in CANDIN is not currently approved for the treatment of common warts or any other indications, but we are exploring whether this biologic product may provide an opportunity to leverage the body’s immune response to more effectively address that condition. Nielsen BioSciences has completed a Phase 2 clinical trial, and we are currently planning for a pivotal Phase 3 study anticipated to begin later in 2021 for this indication.
This program is investigational: CANDIN is not currently approved for the treatment of common warts or any other indication.
Information on our ongoing clinical trials can be obtained at ClinicalTrials.gov/NCT02393417
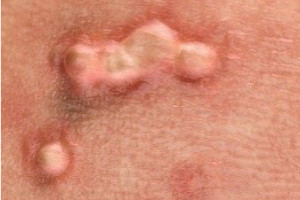
Results in Phase 2 research involving common and non-common warts among adults were highly encouraging, and we are now advancing clinical research into use of the antigen in common and non-common warts. Though not treated, Plantar, Periungual, Flat, Facial, and Genital warts were evaluated in the Phase 2 subjects.
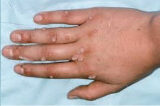
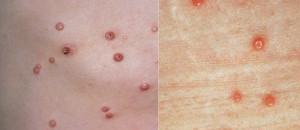
In addition, based on the Phase 2 results and a significant body of peer reviewed publications, we plan to continue evaluating the Candin antigen active ingredient as a potential treatment for a variety of other viral dermatological conditions, including Molluscum Contagiosum, Plantar Warts, Periungual Warts, Flat Warts, Facial Warts, and Genital Warts
Drug Development Pipeline